A round of copper cylinders and zinc sheets is how the Italian physicist Alessandro Volta began producing batteries in 1800 with his Voltaic Pile. Ever since, batteries have changed a lot in their form, size and how they work. You can find them in almost everything — digital devices, road vehicles, household equipment and even in technology built for space flights. In this part of the article, we will concentrate on home inverter batteries.
Home batteries, which are used for energy storage and backup power, come in several main types:
- Lead-acid batteries
- Lithium-ion batteries.
- Flow batteries
1) Lead-Acid Batteries
The lead-acid battery, invented in 1859 by French physicist Gaston Planté, is the oldest type of rechargeable battery still in use today. It operates using lead-based electrodes and a sulfuric acid electrolyte.
Lead-acid batteries function through electrochemical reactions between lead compounds and sulphuric acid. They consist of cells made up of positive and negative plates separated by a permeable membrane to allow ionic flow.
Examples: Flooded, AGM (Absorbent Glass Mat), and Gel lead-acid batteries.
Pros: Relatively inexpensive, widely available, and reliable.
Cons: Lower energy density, shorter lifespan compared to lithium-ion, and limited depth of discharge (not designed to be fully discharged repeatedly).
Applications: Car batteries, backup power systems, and some small-scale solar systems.
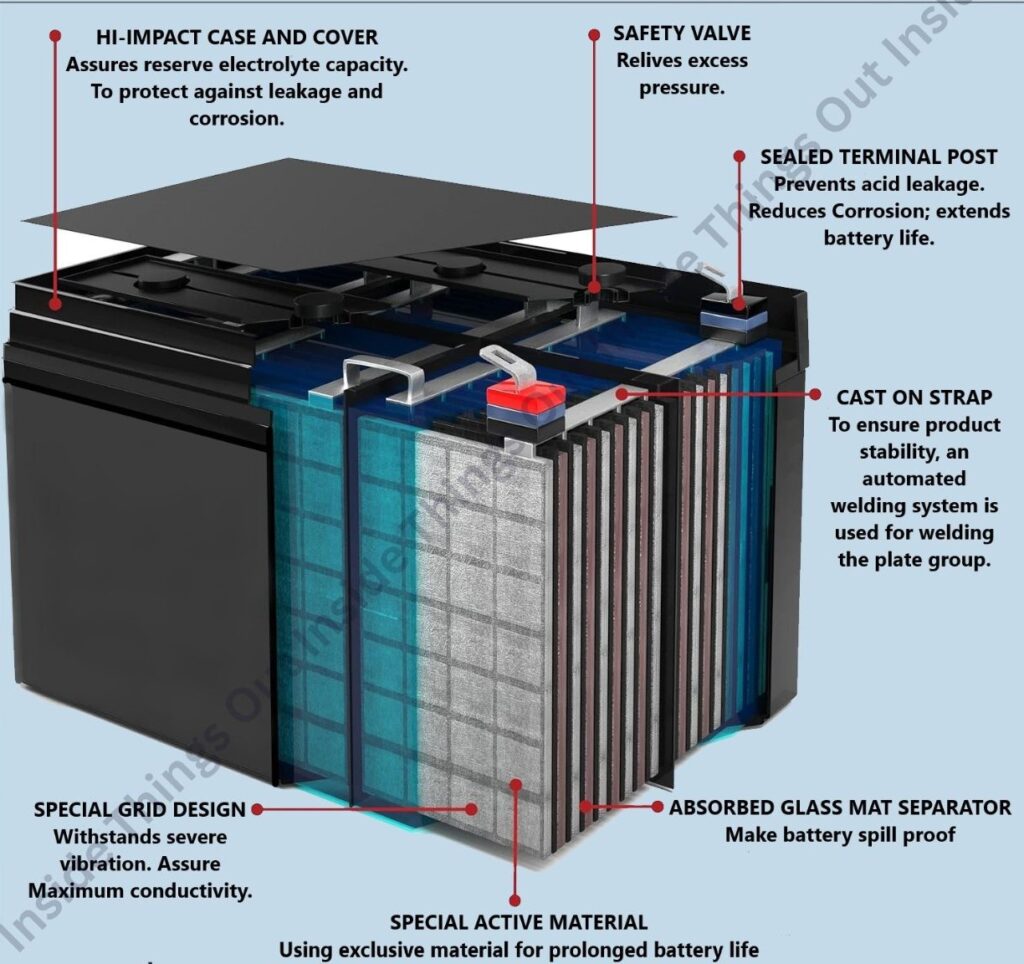
“A lead-acid battery contains plates that serve as electrodes, enabling the battery to charge and discharge by facilitating electrochemical reactions.
Battery Plates
Electrochemical energy storage is possible only with the use of battery plates. Battery plates perform the function of both positive and negative electrodes. They hold the material that is responsible for storing energy in chemistry. They keep the active material used to store and provide energy which is important for setting the battery’s capacity, efficiency and how long it will last.
Two plates with opposite charges are what create a cell. Consequently, groups of cells make up the complete device. The cells in a cellulose membrane are separated so that no physical wetting occurs, while ions can still pass through. These electrodes are capable of storing more than just electric storage. That’s how wires are linked to the terminals, so electricity can flow from them and use an external device or device.
Types of Battery Plates
Battery cell plates, or electrodes, are referred to by their polarity. As such, we have the positive and negative plates. These represent the cathode and anode electrodes, respectively. Here’s more about them:
A) Negative Plates (Anode)
- Undergo oxidation (lose electrons) during discharge.
- Release electrons to the external circuit, powering connected devices.
B) Positive Plates (Cathode)
- Undergo reduction (gain electrons) during discharge.
- Accept positive ions, driving the electron flow through the external load.
Battery Plate Designs
Battery plate design affects the performance, efficiency, and longevity of the battery. Three common designs are:
1. Flat Plates
- Rectangular and flat in shape
- Simple construction
- May include surface textures or a basic grid to increase active material retention
- Found in basic applications.
- These batteries generally provide a shorter cycle life, typically ranging from 600 to 800 charge-discharge cycles.

2. Grid Plates
- A sub-type of flat plates with a honeycomb or mesh-like structure
- Offer higher surface area
- Enhance active material retention and energy storage
- Common in standard lead-acid batteries
- This battery plates typically last 500–1000 cycles.

3. Tubular Plates
- Cylindrical or tube-shaped
- Increased surface area allows more active material and longer discharge cycles
- Ideal for deep-cycle applications and heavy-duty use.
- These battery provides longest cycle life, can last anywhere from 1200 to 1500 cycles depending on usage conditions

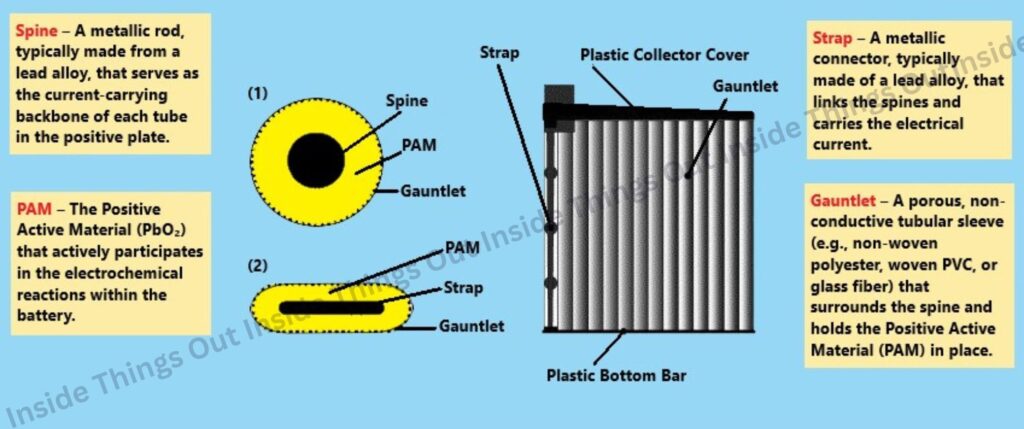
Internal Components of a Tubular Battery
2) Lithium-Ion Batteries:
Lithium-ion batteries were invented in the 1970s; yet, the first models could not be charged. During the 1980s, rechargeable lithium-ion batteries were created which made them a common choice for many industries. At present, most large businesses rely on lithium batteries.
Why Lithium?
When measured by electrochemical potential or energy carry per kilogram, lithium is the leader of all metals. For these reasons, Li-ion is the perfect choice for batteries because it saves space while allowing for plenty of energy storage.
Examples: Lithium Nickel Manganese Cobalt Oxide (NMC), Lithium Nickel Cobalt Aluminum Oxide (NCA), and Lithium Iron Phosphate (LiFePO4).
Pros: Higher energy density, longer lifespan, faster charging and discharging, and more efficient than lead-acid.
Cons: Higher cost than lead-acid and some chemistry have safety concerns.
Applications: Mobile devices, electric vehicles, and residential solar energy storage systems.
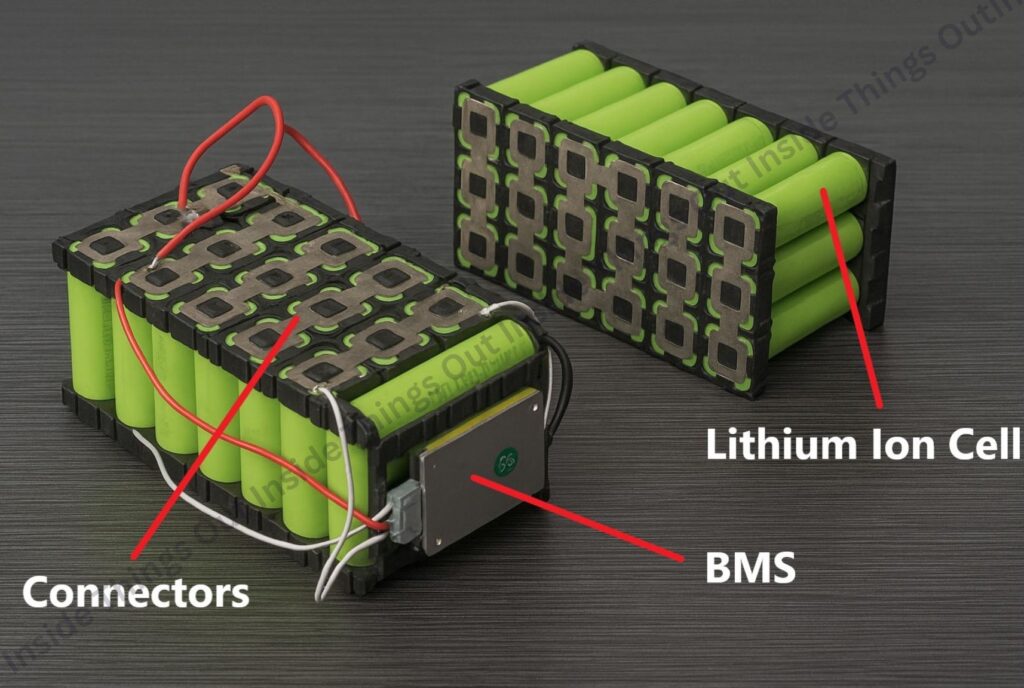
Inside a Lithium-Ion Battery Pack
A lithium-ion battery pack consists of several integrated components that work together to store and deliver electrical energy safely and efficiently. The main elements include:
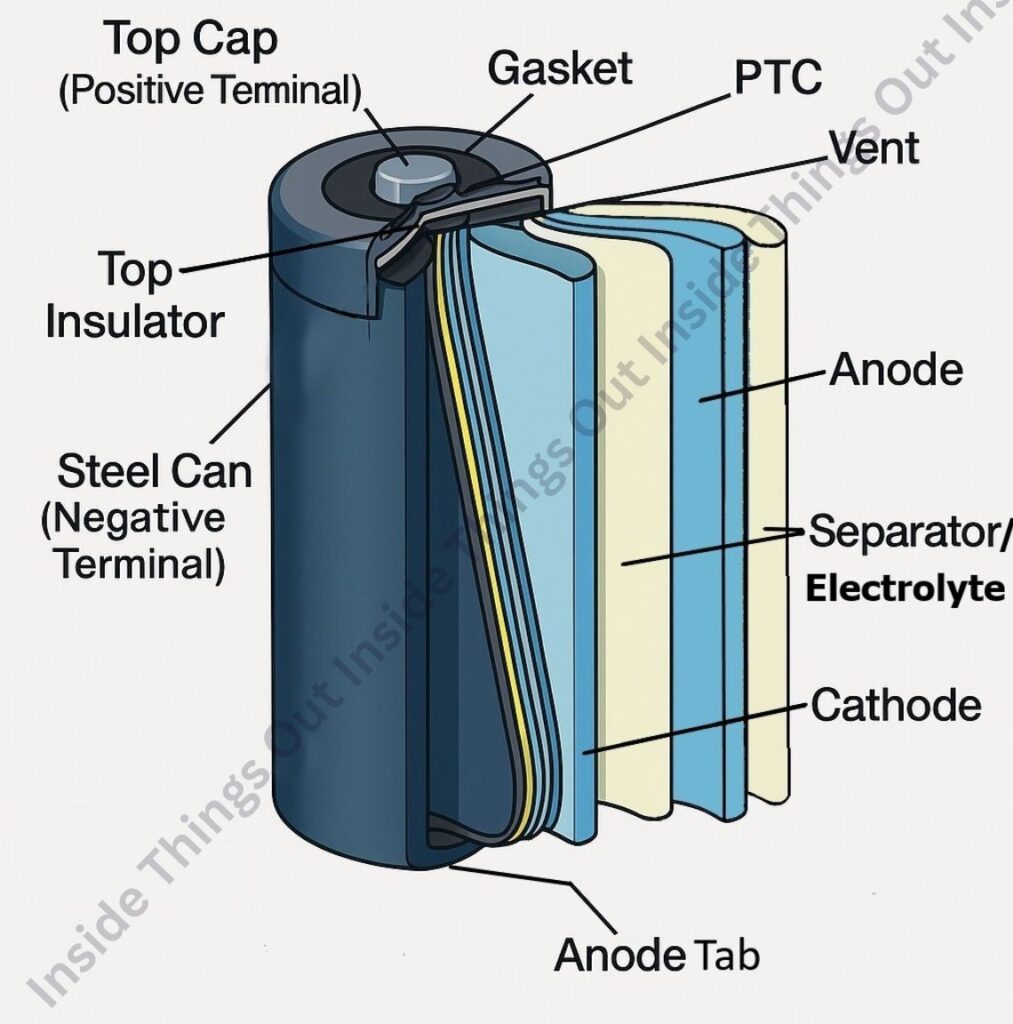
A) Battery Cells
Each lithium-ion cell contains essential components that store and deliver electrical energy efficiently and safely. These include:
i. Cathode (+) – Positive Electrode
- Material: Typically a lithium-containing metal oxide.
- Common Types:
- Lithium Cobalt Oxide (LiCoO₂)Lithium Iron Phosphate (LiFePO₄)
- Nickel Manganese Cobalt Oxide (NMC)
- Function: Releases lithium ions during discharge and accepts them during charging.
ii. Anode (−) – Negative Electrode
- Material: Usually graphite or silicon-based composites.
- Function: Stores lithium ions during charging and releases them during discharge by allowing intercalation within its structure.
iii. Electrolyte
- Composition: Lithium salt (like LiPF₆) dissolved in an organic solvent.
- Function:
- Conducts lithium ions between the cathode and anode.Helps maintain cell efficiency by minimizing internal resistance.
- Lithium travels through a separator sheet soaked in electrolyte.
iv. Separator
- Material: Microporous plastic film (e.g., polyethylene or polypropylene).
- Function:
- Physically separates the cathode and anode.
- Prevents short circuits while allowing ionic conductivity.
B) Modules
- Cells are grouped into modules to facilitate scalability and management.
- Modules are connected in series and/or parallel to achieve the desired pack voltage and capacity.
C) Battery Management System (BMS)
- It measures voltage, temperature and current at the level of the individual cell and the whole module.
- Locks the charging/discharging process to ensure both safety and long life.
- Prevents both overcharging and overdischarging of the cells.
- Senses electrical problems and may split the pack into separate sections if required.
D) Connectors
- Allow electricity to travel through, between and among the battery’s modules and cells, as well as to the BMS.
- Connect using low resistant, reliable cables.
E) Fuses and Contactors
They prevent accidents caused by brief, powerful electrical flows. Should an error or shutdown occur, the contactors will disconnect the battery.
F) Cooling Systems
- Used to regulate temperature, especially in high-demand applications like electric vehicles.
- Can be air-cooled or liquid-cooled, depending on performance requirements
G) Auxiliary Components
It includes:
- Thermal management systems
- Mechanical housings
- Insulation and shock-absorbing structures
- Sensors and wiring
3) Flow Batteries
A flow battery, also known as a redox flow battery, is a type of rechargeable battery in which chemical energy is stored externally in liquid electrolytes. These electrolytes contain electroactive species that participate in reduction-oxidation (redox) reactions within an electrochemical cell to store and release electricity.
Examples: Vanadium redox battery, zinc-bromine battery
Pros: Long lifespan, high energy capacity, and can be discharged to 100%.
Cons: High cost, larger size and weight, and require ongoing maintenance.
Applications: Large-scale energy storage, grid-scale energy storage, and potentially some residential applications in the future.
Key Components and Operation:
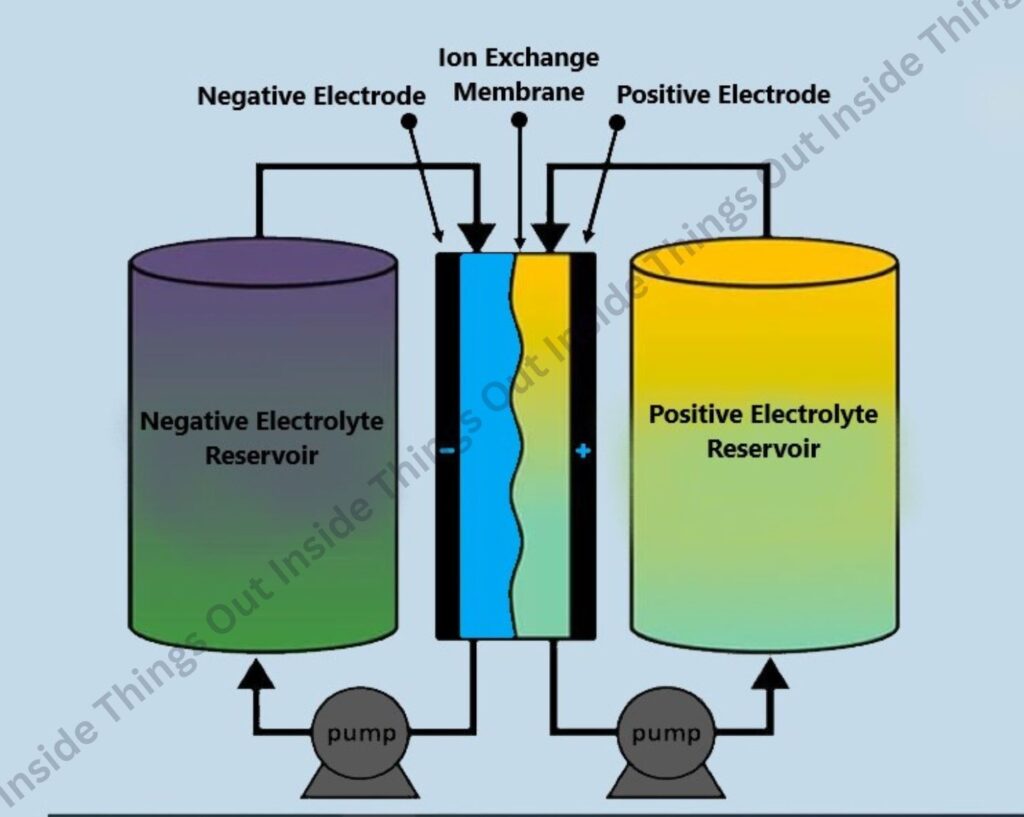
i) Electrolyte Storage Tanks
The chemical energy is stored in two separate tanks, each containing a different liquid electrolyte. These electrolytes house the redox-active materials.
ii) Pumping System
A system of pumps circulates the electrolytes from the storage tanks through the electrochemical cell.
iii) Electrochemical Cell and Redox Reactions
In the electrochemical cell, redox reactions take place:
- During Discharge: The electroactive species undergo redox reactions that release electrons, generating electricity.
- During Charging: An external power source drives the redox reactions in reverse, storing energy in the electrolytes.
In conclusion, much has changed in batteries since Volta invented them. Today, different kinds of home inverter batteries such as lead-acid, lithium-ion and flow batteries, can be used for different requirements. Find that lead-acid is less expensive and dependable, as opposed to lithium offers greater efficiency and longer usefulness and flow batteries make large, flexible storage possible. The suitable solar panel depends on how much electricity you require, your expenditure and the type of use.
*****

